
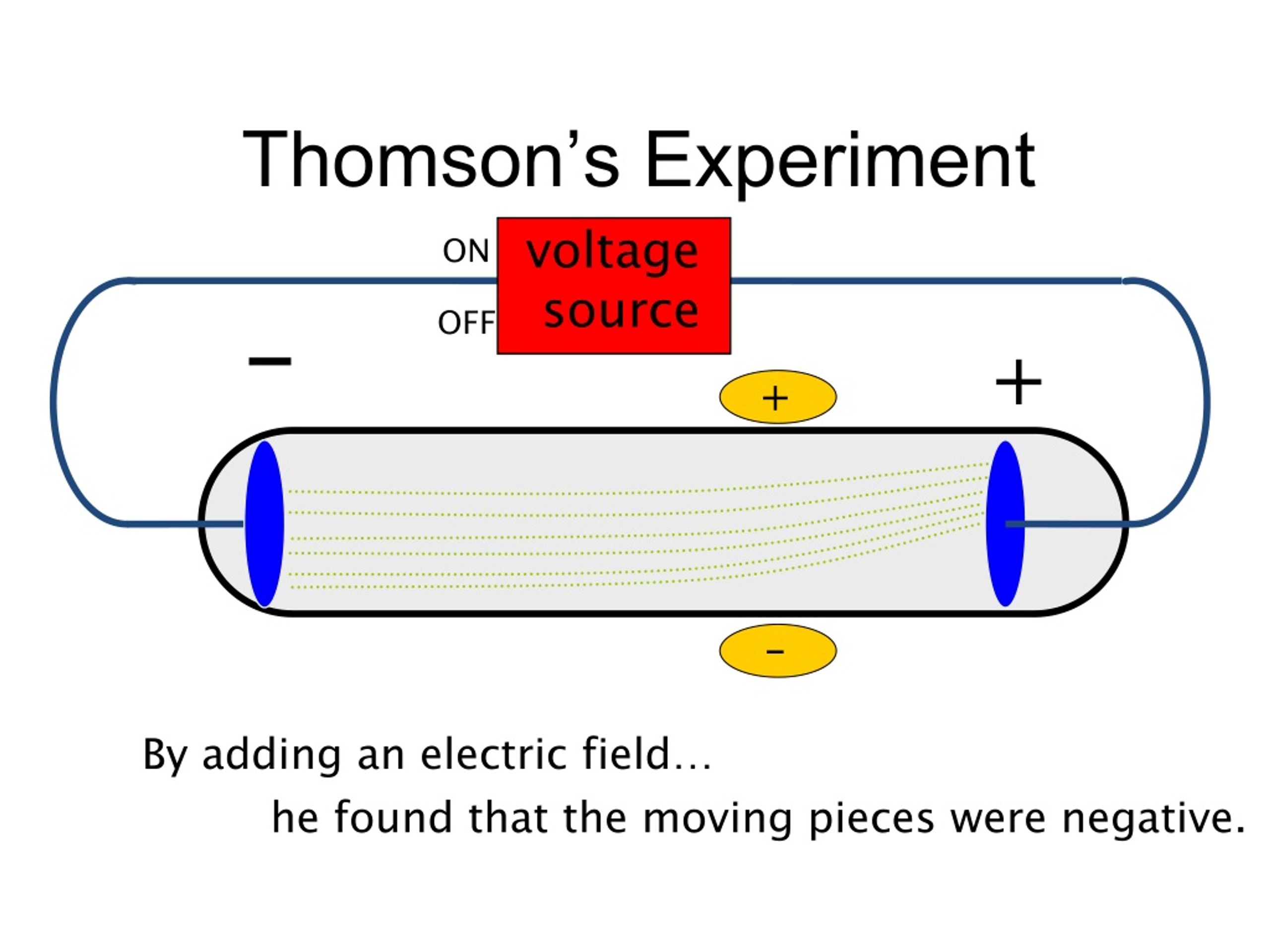
Cathode ray ionises the gases through which they travel.Cathode ray can penetrate through thin metallic sheets.Cathode ray cause green fluorescence and glass surface, i.e., the glass surface on which the cathode rays strike show a green coloured shine.This causes a rise in the temperature of the object. When these particles strike an object, a part of the kinetic energy is transferred to the object. The cathode ray particles possess kinetic energy. Cathode ray heat the object on which they fall.Cathode ray also get deflected when these are subjected to a strong magnetic field.We know that a positively charged body would attract only a negatively charged body, therefore, the particles of cathode rays carry negative charge. When the cathode rays are subjected to an electric field, these get deflected towards the positively charged plate (anode). Cathode ray consists of negatively charged particles.This is due to the impact of the particles of the cathode rays on the blades of the paddle wheel. Cathode ray sets a paddle wheel into motion when it is placed in the path of these rays. Cathode ray consists of matter particles and possess energy by the virtue of their mass and velocity.

The path cathode ray travel is not affected by the position of the anode. That is why, cathode rays cast a shadow of any solid object placed in their path. What are the properties of cathode rays?Ĭathode ray show the following properties: Therefore, these rays are called cathode rays. This fluorescence of walls is due to the bombardments of glass by rays emitted from the cathode. This is due to a phenomenon called fluorescence. Structure of atomĪt this stage, the glass (walls) off the discharge tube, opposite to cathode, starts glowing with a faint greenish light. The emission of these rays in a discharge tube is shown in figure. These radiations are called cathode rays. When a high very high electric potential, ~10000 V, is applied across a gas taken in a discharge tube at very low pressure, about 10 -5Īdvertisement atm, some radiations are emitted from the cathode. Cathode Rays How are cathode rays produced?
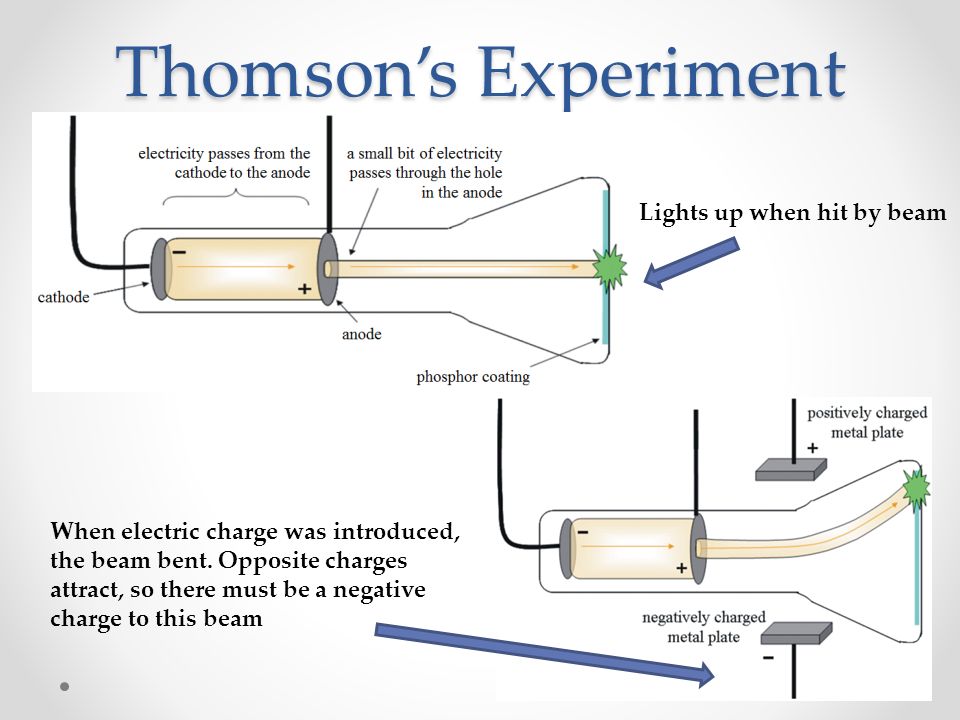
Thus, we see that when electric discharge is passed through gases at very low pressures, cathode rays are produced. At this stage, a stream cold cathode rays are emitted from the cathode. But the walls of the discharge tube opposite the cathode start glowing with light greenish light. At 10 -5 atm pressure: When the pressure is lowered to about 10 -5 atm, light emission by the residual air in the discharge tube stops.The colour of the glow depend upon the nature of gas in the tube, and on the colour of the gas used for making the discharge tube. At 10 -3 atm pressure: At sufficiently low pressure, this glow fills the whole tube.At this stage, electric current begins to flow from one electrode to the other electrode. At 10 -2 atm pressure: At about 10 -2 atm pressure, a glow surrounding the cathode, negative electrode, leaves the electrode surface, and little space is left between it and the electrode.When the pressure inside the discharge tube was reduced gradually, the following observations were made:
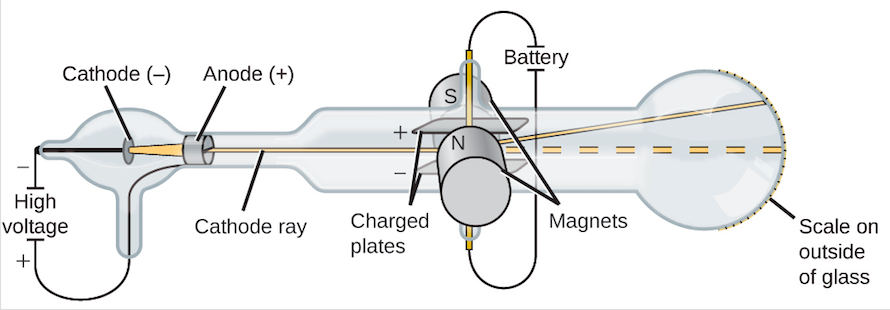
But it was discovered by William Crookes that gas could conduct electricity at low pressure. It was found that gases do not conduct electricity even when an electric potential of about 10000 V is applied. 80 Conceptual Questions of the P-Block Elements Class 12


 0 kommentar(er)
0 kommentar(er)
